ICARUS
Immune Checkpoint Antagonism to RedUce Squamous cell carcinoma
Survey Questions
REDCap Survey
https://redcap.link/k77y3o9w
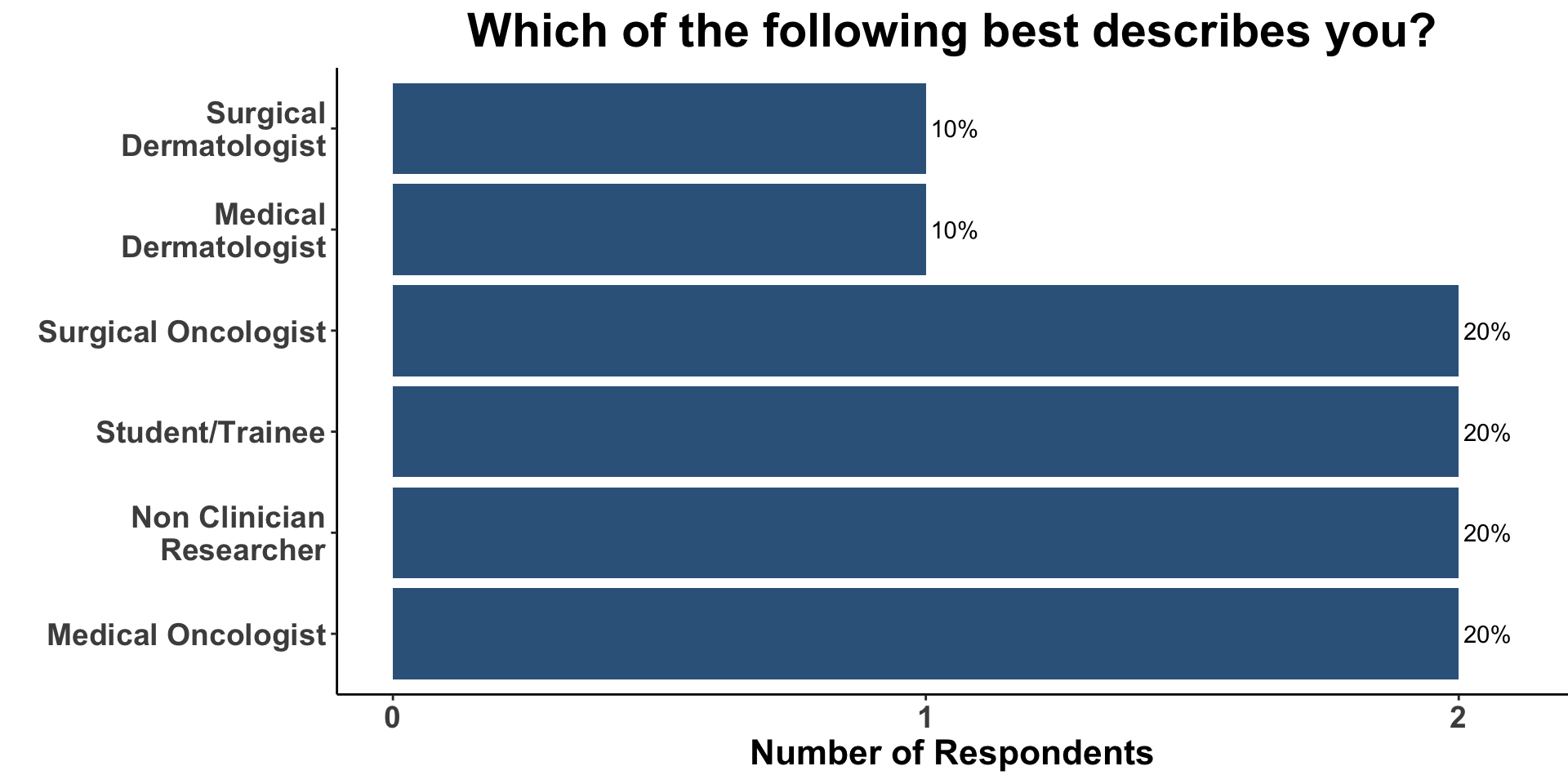
Role
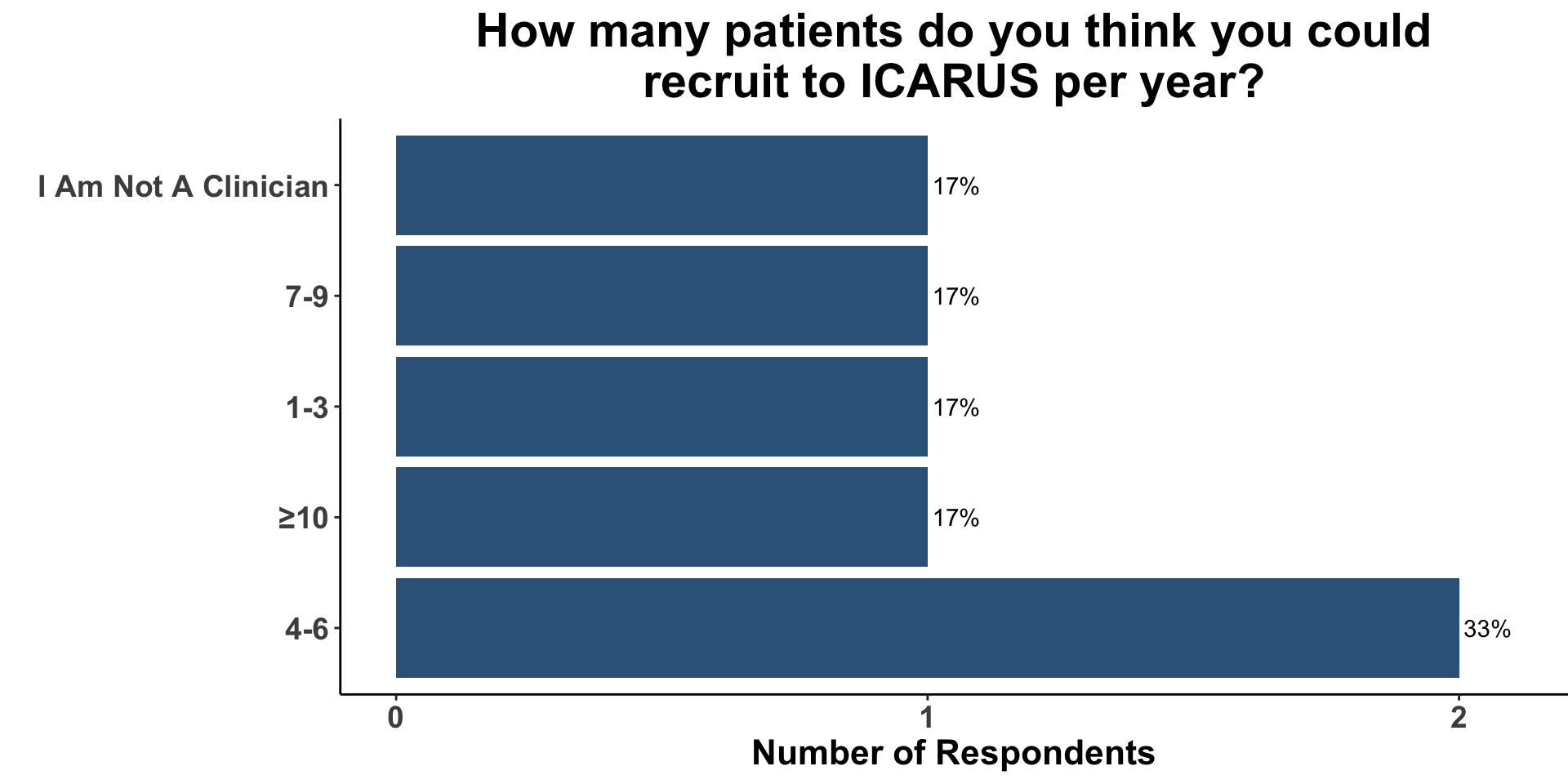
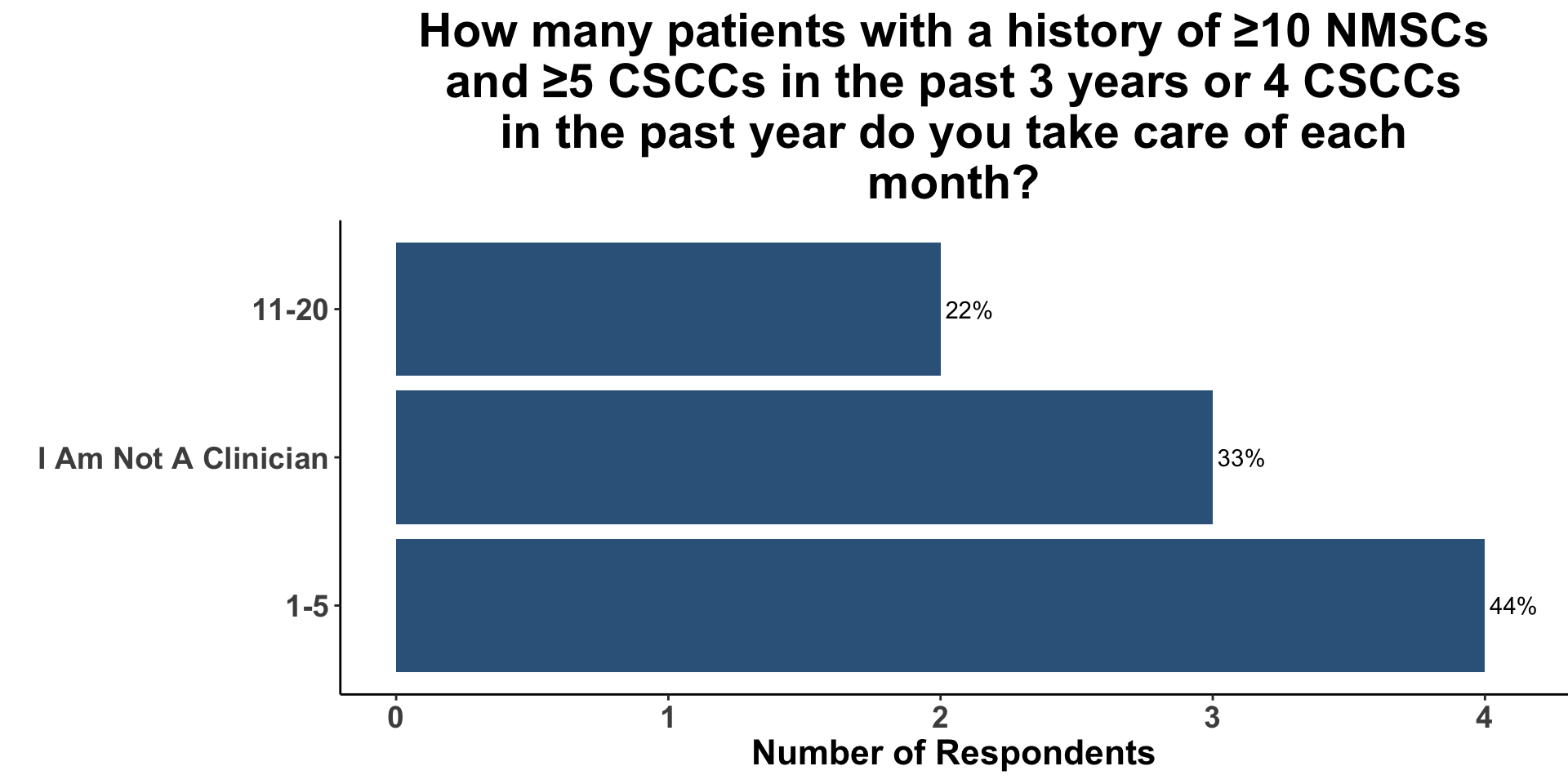
CSCC Patient Care Experience
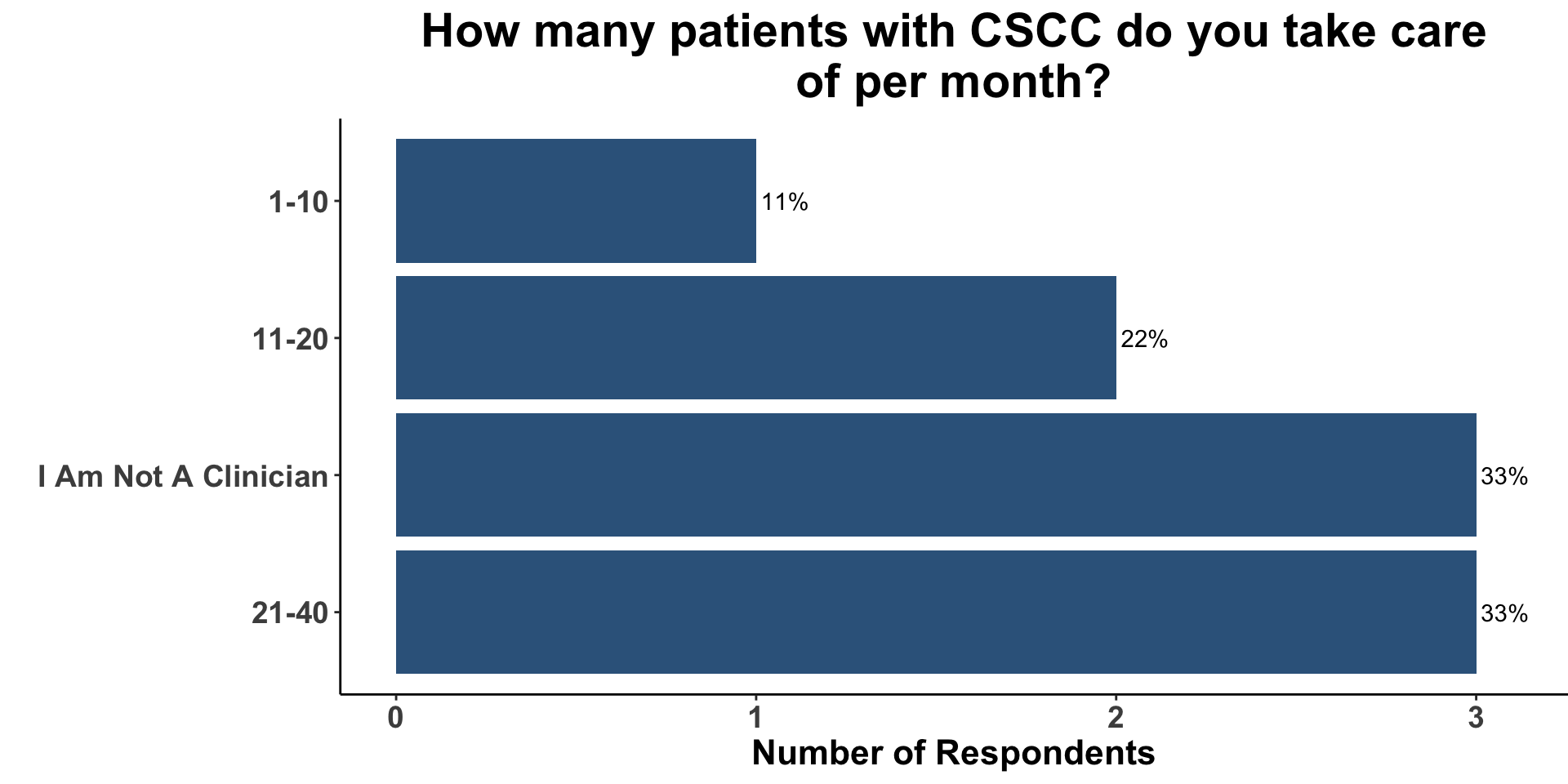
CSCC Patient Care Experience
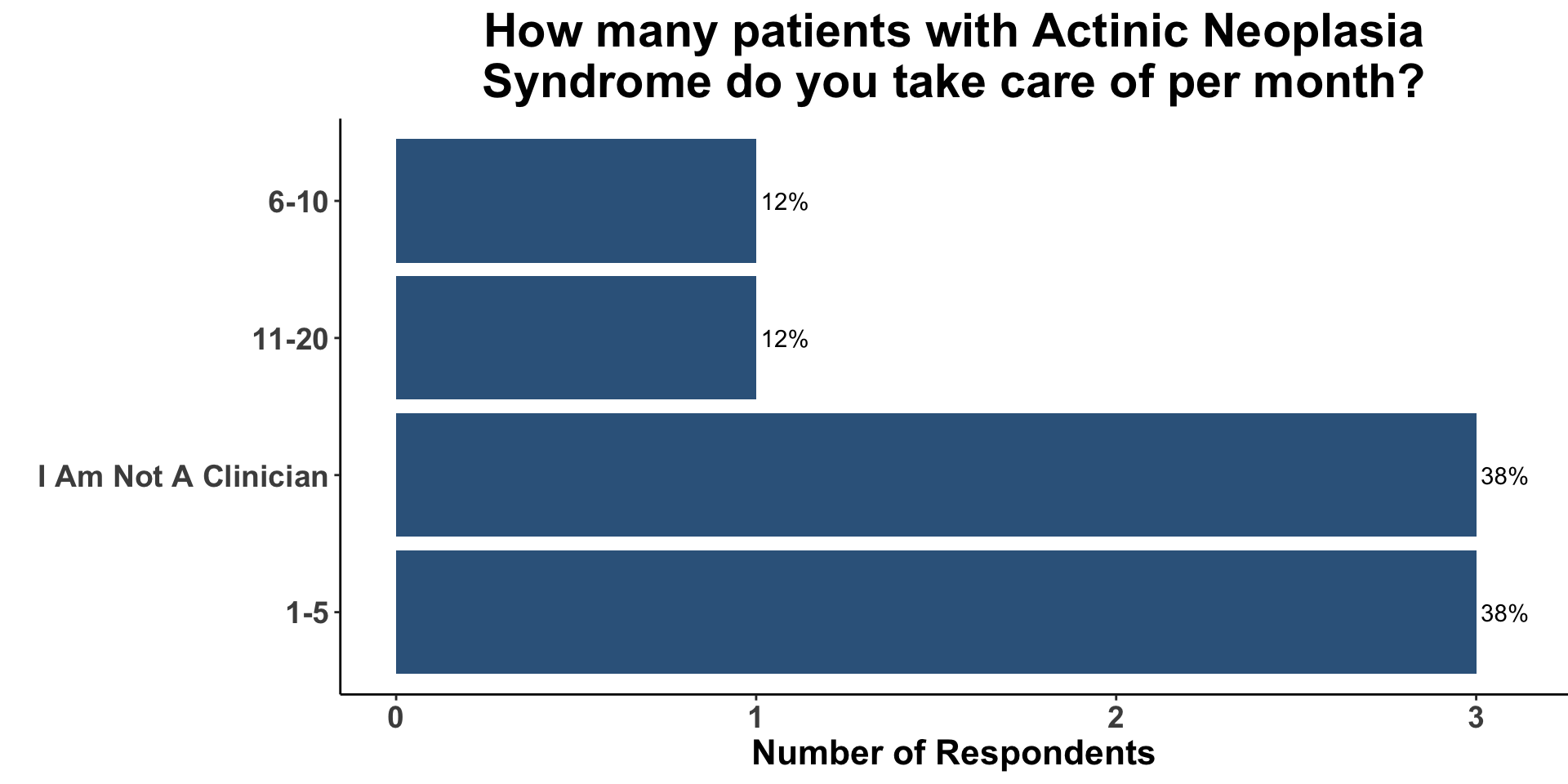
CSCC Patient Care Experience
Overview
- Hypothesis
- Rationale
- Schema
- Schema rationale
- Efficacy Assessment
- Statistical analysis
- Sample Size Calculations
- Eligibility Criteria
- Treatment Considerations
- Alternative Approach
Hypothesis
- Anti-PD1 therapy will decrease the development of CSCC in high-risk patients with a history of multiple NMSCs
Rationale
- Effective preventive strategies to mitigate CSCC are lacking
- Anti-PD1 therapy has shown to be effective in the neoadjuvant and advanced setting
- Intervention earlier in the disease journey will improve outcomes for patients at high-risk for morbid disease
Schema - Rationale
- Feasibility
- Patients with ≥10 NMSCS (≥5 CSCCs) enriches for patients at high risk for recurrent disease
- Active surveillance for high-risk patients involves q3-6 month TSE (minimizes extra visits)
- Bias
- Placebo
- Important to reduce bias when outcome is determined by physical exam & not scans
- Placebo
- Efficacy Assessment
- Primary Endpoint: Rate of new KCC is an established endpoint (e.g.ONTRAC & ONTRANS (NEJM 2015/2023))
Efficacy Assessment
- Primary endpoint
- Rate of new CSCC
- count data
- Rate of new CSCC
- Modeling count variables
- Poisson regression
- Negative binomial regression
- Similar structure as Poisson, but has an extra parameter to model over-dispersion
- When the observed variance is higher than the variance of the theoretical model
- Common in real-world data sets are populations are often heterogeneous and non-uniform
- When the observed variance is higher than the variance of the theoretical model
- Similar structure as Poisson, but has an extra parameter to model over-dispersion
Efficacy Assessment
library(MASS)
glm.nb(
formula = `New CSCCs` ~ Treatment + `Baseline CSCCs` + `CLL` + `Previous Radiation`,
init.theta = 0.75
)Secondary Endpoints
- Number of new basal-cell carcinomas & actinic keratoses during the 12-month intervention period
- The number of new non-melanoma skin cancers in the 6-month post intervention period
- Safety of anti-PDX as assessed by the numbers and types of adverse events
Sample Size Calculations
- Considerations
- Sample size will vary depending on:
- baseline event rate
- effect size
- baseline event rate
- Sample size will vary depending on:
Sample Size Calculations
- Example #1
Total Sample Size: 254
Sample Size Calculations
- Example #2
Total Sample Size: 299
Sample Size Calculations
- Example #3
Total Sample Size: 348
Sample Size Calculations
- Example #4
Total Sample Size: 172
Sample Size Parameters
Sample Size Parameters
Background on NMSC Incidence
- Risk of subsequent NMSC is proportional to numbers of previously diagnosed skin cancers
- In one study, the five year risk of developing another skin cancer was estimated at >60% for individuals with two previous skin cancers and at >90% for individuals with 4-5 previous skin cancers(Karagas 1992)
- In ONTRAC, patients receiving placebo developed on average 2.4 NMSCs (0.7 CSCCs) in 12 months(Chen et al. 2015)
- Inclusion: ≥2 NMSCs in the previous 5 years
- Actual Baseline: 7.9 ± 8.0 NMSCs, 2.1 ± 3.5 CSCCs in Placebo
- In ONTRANS, patients receiving placebo developed on average 2.7 NMSCs (1.9 CSCCs) in 12 months(Allen et al. 2023)
- Inclusion: ≥2 NMSCs in the previous 5 years
- Actual Baseline: 7.5 ± 7.6 NMSCs, 4.8 ± 5.6 CSCCs in Placebo
Eligibility Criteria
- Inclusion
- At least 10 NMSC, ≥5 CSCCs
- ECOG 0, 1 or 2
- Exclusion
- Previous ICI
- Active pharmacological immunosuppression
- HIV with detectable viral loads (undetectable dz allowed)
- Field treatment with the past 4 weeks
- Nicotinamide use within the past 3 months
Calculating the sample sizes
[1] 254 259 258[1] 254 259 258[1] 213 216 216[1] 217 221 220 Outcome + Outcome - Total Prevalence *
Exposed + 206 79 285 72.28 (66.69 to 77.40)
Exposed - 210 79 289 72.66 (67.14 to 77.72)
Total 416 158 574 72.47 (68.62 to 76.09)
Point estimates and 95% CIs:
-------------------------------------------------------------------
Prevalence ratio 0.99 (0.90, 1.10)
Odds ratio 0.98 (0.68, 1.41)
Attrib prevalence in the exposed * -0.38 (-7.69, 6.92)
Attrib fraction in the exposed (%) -0.53 (-11.20, 9.11)
Attrib prevalence in the population * -0.19 (-6.50, 6.11)
Attrib fraction in the population (%) -0.26 (-5.40, 4.62)
-------------------------------------------------------------------
Uncorrected chi2 test that OR = 1: chi2(1) = 0.011 Pr>chi2 = 0.918
Fisher exact test that OR = 1: Pr>chi2 = 0.926
Wald confidence limits
CI: confidence interval
* Outcomes per 100 population units est lower upper
1 2.607595 2.263646 2.989036
2 2.658228 2.310837 3.043103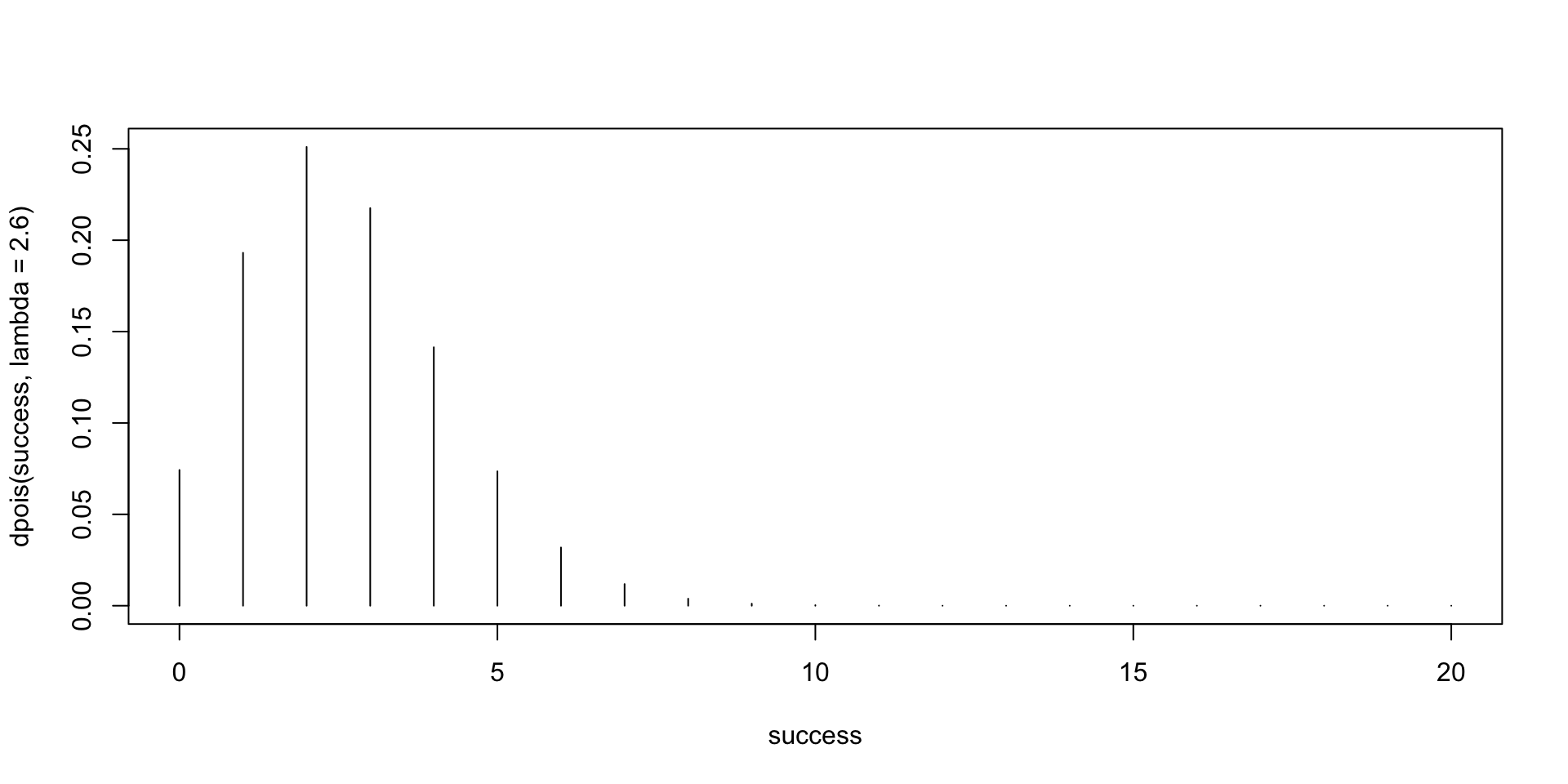
Exact Poisson test
data: 5 time base: 3
number of events = 5, time base = 3, p-value = 0.2345
alternative hypothesis: true event rate is not equal to 1
95 percent confidence interval:
0.5411621 3.8894440
sample estimates:
event rate
1.666667
Two-sample Negative Binomial rates Tests (Equal Sizes)
N = 175.1805
mu1 = 4.9
mu2 = 7
theta = 0.8
duration = 1
sig.level = 0.05
power = 0.8
alternative = two.sided
NOTE: N is number in *each* groupTreatment Considerations
ICI Antibody Characteristics
- For most ICIs (except ipilimumab), there is no clear relationship between dose and efficacy or safety
- The dose-response and exposure-response curves showed an obvious plateau, possilby implying that increasing doses do not contribute to tumor control
ICI Antibody Characteristics
| ICI Characteristics | ||||
|---|---|---|---|---|
ICI Dose Response
| Dose-Efficacy | ||||||
|---|---|---|---|---|---|---|
ICI Dose Response
| Dose-Efficacy | ||||||
|---|---|---|---|---|---|---|
Background
To model count data, we can use a poisson distribution, as count data are not continuous (they’re whole numbers), and thus a poisson regression. However, Poisson regression assumes that the variance of y equals the mean of y. A dispersion parameter of 1 means that that mean = variance. However, in many real-world data sets, the variance is greater than the mean, and this is called “overdispersion”. A hint of this is if the residual deviance in your model is equal to the degrees of freedom. Dispersion Parameter == Sum of Squared Pearson Residuals / df. Overdispersion can result in underestimation of the standard error (more likely to have a false positive). Causes of overdispersion include predictor variables that are not included in the model; clustering or heterogeneity in the sampled population.
Negative binomial model is a poisson model but allowing parameter k. Variance = mean + mean2/k. Only works for overdispersion, not underdispersion. (can also choose a quasi-poisson)
Alterative Approach
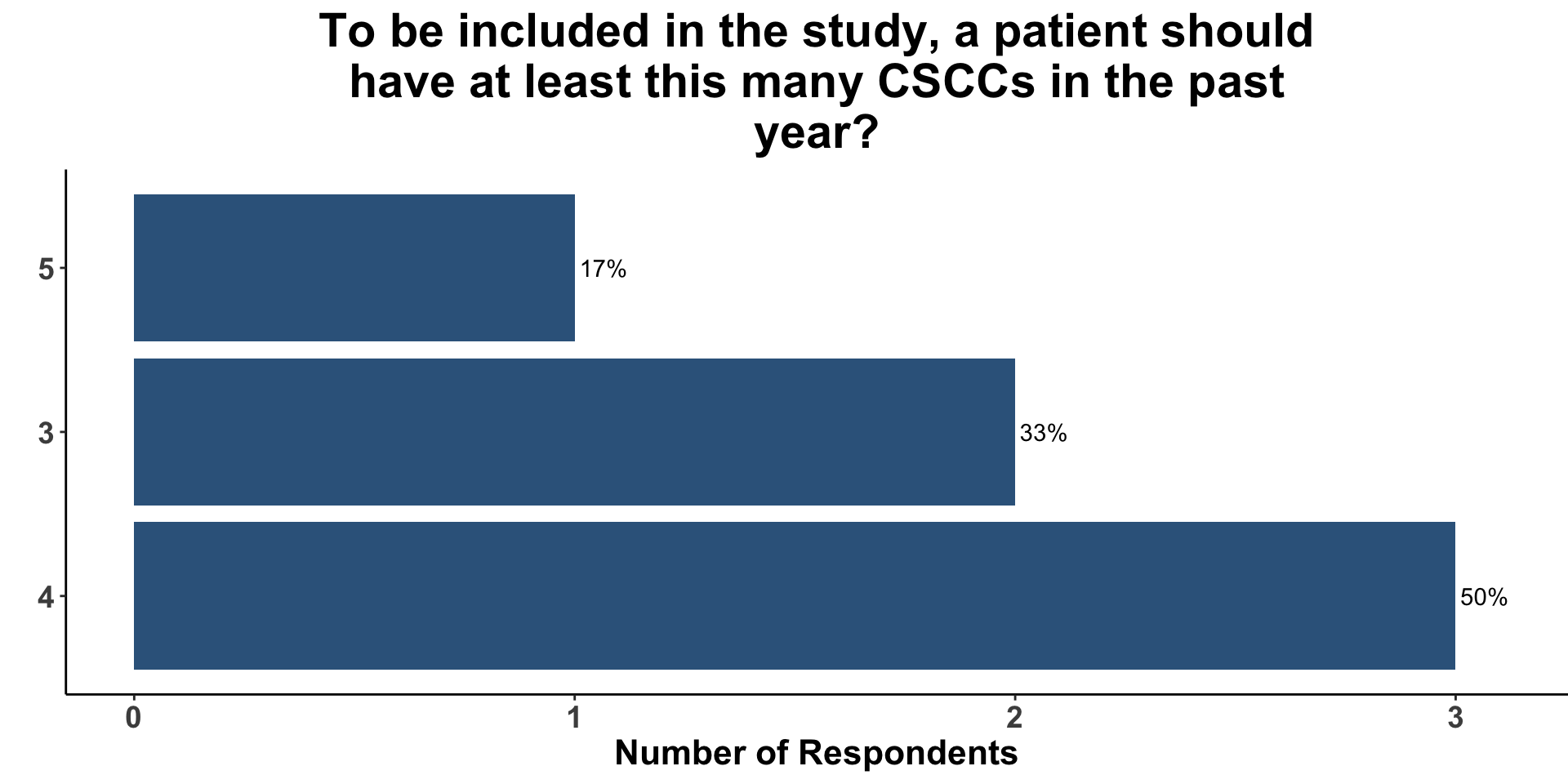
Inclusion Criteria
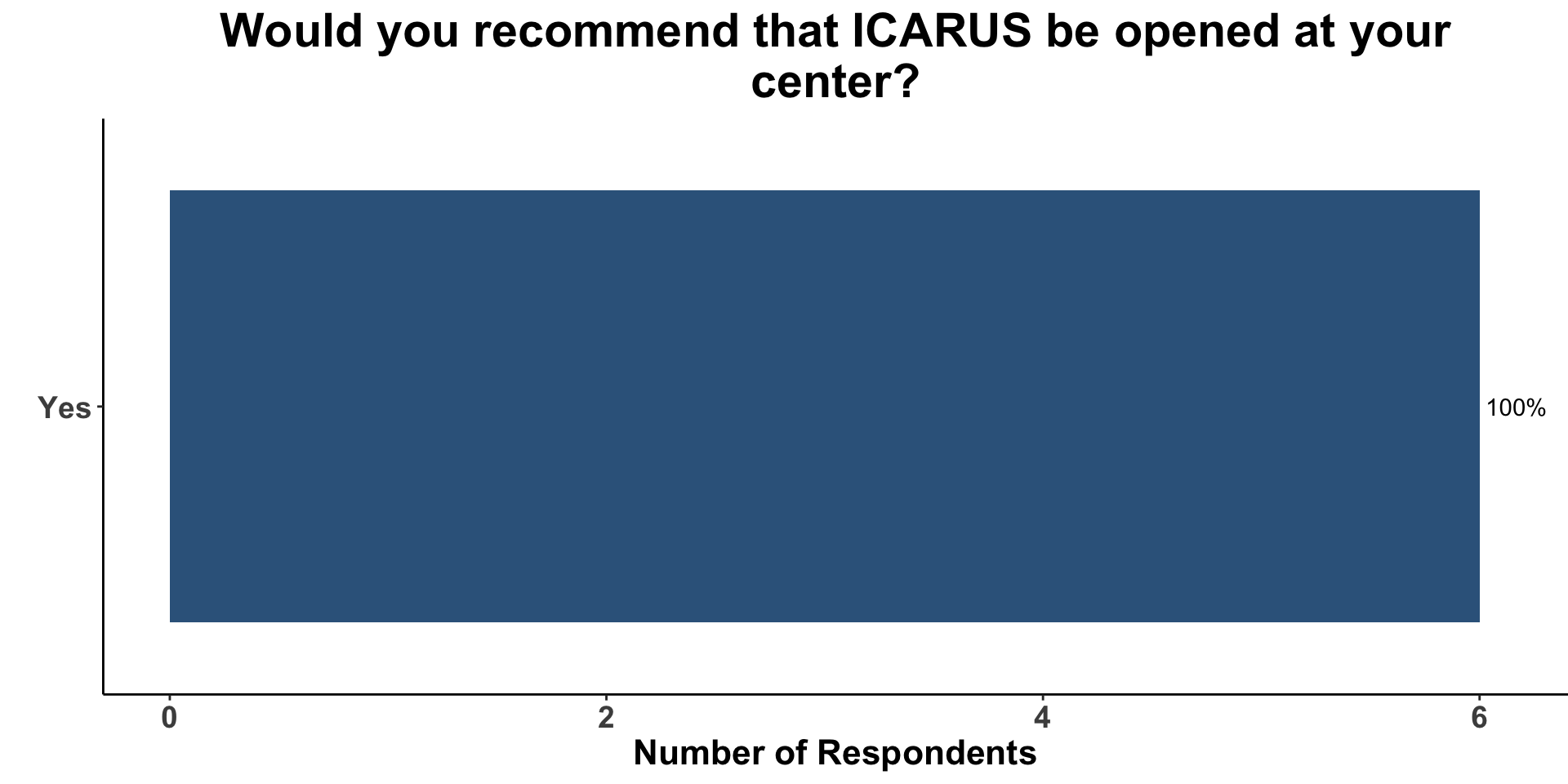
Open ICARUS
Inclusion Criteria